![]() |
|
![]() |
|

Methods of Analysis
The analytical methods described below are the most common of those performed on calcium chloride products. Other methods might be used for specific tasks and analyses.
The descriptions in the linked pages below should be considered only as overviews and should not be utilised as reference material for laboratory work.
| Calcium Chloride Analysis |
|
![]() |
For calcium chloride (CaCl2) the concentration is determined by analysing the chloride content by potentiometric titration with silver nitrate according to the following equation:
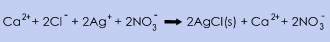
The concentration of silver ions is followed by a sliver electrode. The analysis is carried out in acidic aqueous solution. Concentration of CaCl2 in the original sample is then calculated from the chloride concentration.
| Normal Measuring Range |
30-100% CaCl2 |
| Quantification Limit |
10% |
| Tolerance |
+/- 0.9% (95% confidence interval) |
An alternative approach is to utilise EDTA-titrations with a suitable indicator and visual endpoint determination.
| Na, Mg, K, Fe, Pb Analysis |
|
![]() |
Sodium, magnesium, potassium and lead are determined by ICP-ES (Inductively Coupled Plasma-Emission Spectroscopy) after digestion of the sample in nitric acid.
| Sodium (Na) |
| Normal Measuring Range |
0.01 - 2% |
| Quantification Limit |
0.01% |
| Magnesium (Mg) |
| Normal Measuring Range |
0.002-0.1% |
| Quantification Limit |
0.002% |
| Potassium (K) |
| Normal Measuring Range |
0.002-0.1% |
| Quantification Limit |
0.002% |
| Iron (Fe) |
| Normal Measuring Range |
0.2-1000 mg/kg |
| Quantification Limit |
0.2 mg/kg |
| Lead (Pb)* |
| Normal Measuring Range |
5-1000 mg/kg |
| Quantification Limit |
5 mg/kg |
| *Food Grade only |
ICP-ES can also be used to analyse most other metals in a calcium chloride sample.
An alternative to ICP-ES is AAS (Atomic Absorption Spectroscopy) either with flame or graphite furnace.
| Determining Alkalinity |
|
![]() |
Alkalinity is determined as Ca(OH)2 by titration with hydrochloric acid in aqueous solution. The end point is determined visually by a phenolphtalein indicator. The titration reaction can be written according to the following equation:

| Normal Measuring Range |
0.01-0.5% |
| Quantification Limit |
0.01% |
|
Analysing Insoluble Matter |
|
|
![]() |
Determining Insoluble Matter
Insoluble matter is determined by dissolution of the sample in water and subsequent filtration through membrane filters with pore size 0.2 µm. The insoluble matter is then analysed gravimetrically after the drying of the filters at 105°C.
| Normal Measuring Range |
0.005-1% |
| Quantification Limit |
0.002% |
| Determining pH |
|
![]() |
pH is typically determined in a 10% CaCl2 solution for technical grade calcium chloride and in a 5% solution for food grade calcium chloride. A combined glass electrode is used and the equipment is calibrated daily in the pH range of 4-12.
| Normal Measuring Range |
pH 3-13 |
| Quantification Limit |
minimum pH 3 |
| Determining Heavy Metals |
|
![]() |
Heavy Metals as Lead (FAO Method)
Heavy metals (Ag, As, Bi, Cd, Cu, Hg, Pb, Sb and Sn) are precipitated as their corresponding sulphides using a saturated H2S solution. The turbidity of the sample is compared to a standard containing only lead that is treated in the same way as the sample.
Note: This analysis is only performed on food grade calcium chloride.
| Normal Measuring Range |
Flakes < 20 mg/kg
Solution < 7 mg/kg |
| Quantification Limit |
N/A |
Note: The results should be interpreted as, "less than 20 mg/kg" or "less than 7 mg/kg" of heavy metals as lead." |
Mercury (Hg)
Mercury is determined by AAS (Atomic Absorption Spectroscopy) after dissolution of the sample in nitric acid.
Note: This analysis is only performed on food grade calcium chloride.
| Normal Measuring Range |
0.005-0.10 mg/kg |
| Quantification Limit |
0.005 mg/kg |
|
| Analysing Fluoride |
|
![]() |
Flouride is determined as flouride ion (F-) in aqueous solution using an ion selective electrode (ISE).
Note: This analysis is only performed on food grade calcium chloride.
| Normal Measuring Range |
0.001-1% |
| Quantification Limit |
0.001% |
Arsenic (As) is analyzed by AAS (Atomic Absorption Spectroscopy) using the graphite furnace method. Prior to analysis, the sample is digested in nitric acid.
Note: This analysis is only performed on food grade calcium chloride.
| Normal Measuring Range |
0.05-60 mg/kg |
| Quantification Limit |
0.05 mg/kg |
| Cowin Calcium Chloride Analysis Office Room |
|
![]() |

|
| |
|
|